Quality System
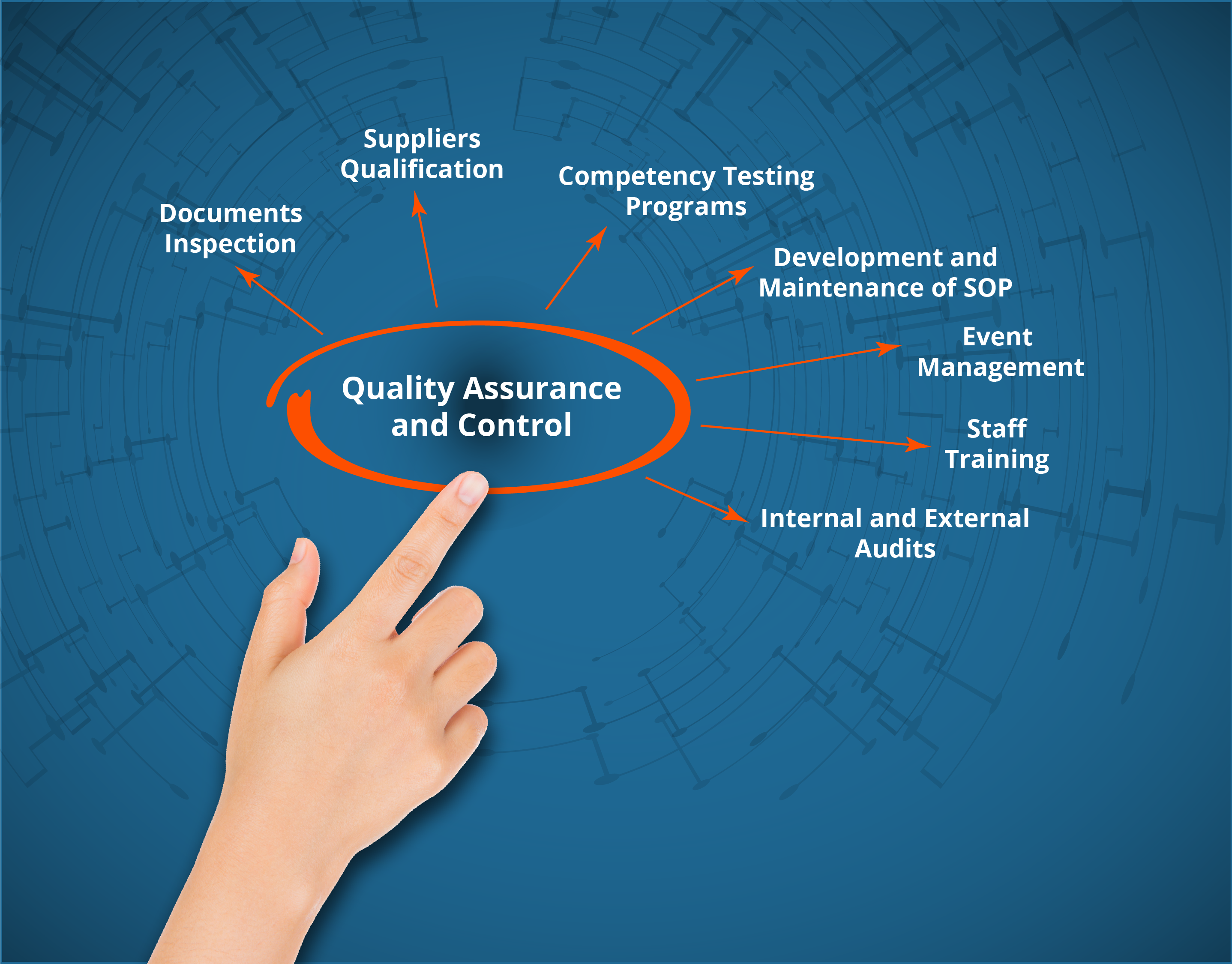
Quality System
Validapro’s Quality Assurance and Quality Control experts can assist you in all your international regulatory compliance projects (FDA, EMA, ANSM, ANVISA, Japan and Health Canada) for pharmaceutical and biologics products, natural health products, cosmetics and medical devices.
Validapro adapts its services according to your needs, from systems evaluation (QA/QC), the implementation of Quality structures to the writing of standardized operating procedures.
Validapro’s team can assist you in:
Analyze your needs according to the regulations
Preparation of trainings adapted to the staff of quality and production services such as GMP, deviation, OOS, etc
Revision or drafting of Quality documents
– Quality manual
– Procedures (Deviation/OOS, Change Control, Complaints, CAPA, Risk Analysis, Recall, etc.)
– Training materials
– GMP/GDP/Annual Product Reviews/Quality Agreements
– Audits (internal, supplier)Development, approval and implementation of Quality Control Systems
– Sampling plan, receiving, storage
– Specifications
– Stability
– Management/preparation of reagents and standards
– Raw Data Management (Data Integrity)
– Batch release tracking and approval
Validapro also offers services
regulatory compliance including:
Supplier audits (Materials, Active Ingredient, Manufacturing, Warehousing, Distribution)
Support for management of complaints, incidents and recalls
Support in the application of Quality standards
Support and temporary replacement of Quality Assurance staff
